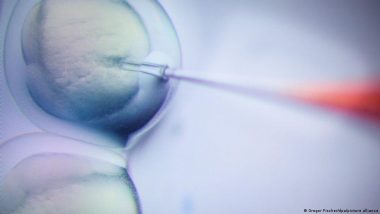
Gene editing in humans has already happened — there are people waking around with CRISPR-Cas9-edited genomes. Right or wrong, they’re here to stay. How do gene therapies work and what are their limits?In 2018, a scientist called He Jiankui shocked the world when he announced the birth of two babies with edited genomes.
Jiankui used the CRISPR-Cas9 gene editing tool on the twins when they were embryos to rewrite their individual CCR5 genes, creating resistance to HIV.
These two children, along with a third gene-edited child born a year later, represent the world's first gene-edited babies. Five years on, all three are reportedly living healthy, normal lives.
The event sparked major outcry among scientists and non-scientists alike, ultimately leading to Jiankui's three-year imprisonment in China for conducting "illegal medical practices."
But although the three children may represent the first cases of embryo-stage genome editing, they aren't the only humans with an edited genome. More than 200 adults have been treated in clinical trials using CRISPR-Cas9 to treat sickle cell disease. Gene editing therapies have completely changed their lives, curing the blood disorder that once disabled them. Right or wrong, new trials are in the pipeline for other genetic diseases.
CRISPR-Cas9 gene editing
The sudden explosion in gene therapies in the last decade comes down to the development of CRISPR-Cas9. While gene editing has existed since the 1990s, CRISPR was so revolutionary because of its precise and programmable way of altering DNA.
CRISPR-Cas9 is a two-part molecular system. CRISPR is the part you can program to find one specific part of DNA in a gene, while Cas9 is the editing part which cuts out a strand of DNA and adds a new piece in.
Sounds simple enough, but the hard part is delivering it — the process must occur in trillions of cells inside us if we want to use it to treat a disease.
As such, CRISPR is only suitable to treat diseases with "simple" genetic mutations in one cell type. If you know with certainty that a disease is caused by one error in one gene, like sickle cell anemia or certain forms of cancer, CRISPR has the potential to treat it.
The limits of gene therapy
What CRISPR can't treat are complex genetic disorders — anything with multiple mutations in multiple genes in different types of cells.
"Very few patients have an identifiable genetic cause [of a disease] that you can target," said David Curtis, a professor of medical genetics at University College London, UK.
Curtis is doubtful whether CRISPR can be used to treat any diseases that have more than one genetic mutation involved, at least for the time being.
"If you take a complex genetic disorder like schizophrenia, gene therapy isn't going to help. The brain is an insanely difficult target to treat — you'd have to make multiple DNA changes in billions of brain cells to rewire brain cells that have developed in the wrong place. This doesn't seem likely to work," Curtis told DW.
Designer babies
There are two main approaches to gene editing: germline editing and somatic cell therapies.
Germline editing is performed during embryonic development. If you edit the embryo when it's a single or small number of cells, you edit all the cells that will divide from them, meaning the gene edit is in all the cells of the body.
Great for curing diseases, but the kicker is that this includes sperm and egg cells, meaning the edited gene is passed down to children and grandchildren. So by editing embryos, you alter the course of evolution for generations after. The use of this method, which sits at the heart of the "designer baby" debate, put Jiankui in jail.
Advocates say we could ensure none of our kin develop diseases like HIV/AIDS or multiple forms of cancer, essentially eradicating them from existence.
On the other hand, there are the problems of playing God. More on this later.
Somatic cell therapies have potential
In somatic cell therapy, genes are edited in cells removed from the patient (or donor stem cells), then returned to the patient. It's the method that is being used to treat sickle cell anemia and has potential for the treatment of multiple other genes with single point mutations.
Most scientists argue that somatic cell therapies have much more potential than germline editing. We've seen that it can work, and it sidesteps the awkward issues of designer babies.
But somatic cell therapy has its problems, the most serious being potential off-target effects on the genome.
"There's a chance you can introduce mutations into unwanted sites of the genome when you use CRISPR, changing or inactivating the function of other genes. The effects of this on the body are not really known," said Van Trung Chu, a CRISPR scientist working at the Max Delbrück Center for Molecular Medicine in Berlin.
Some random mutations in the genome could be harmless, but others could also run the risk of causing cancer or other genetic disorders. There are ways of combing the genome to look for mutations, but it's unclear yet what would happen if mutations were found. More gene editing to fix the first gene editing errors?
"The mutations aren't completely random. Depending what Cas gene editing tool you use, you have an idea of where those mutations are likely to be," Chu told DW.
All scientists can do now is perform deep sequencing analysis of genomes to find potential orthogonal mutations. It's a bit of a needle in a haystack situation, but you know roughly in which part of the haystack to look.
"In the long-term, new forms of CRISPR-Cas9 technologies are being created that have less potential for off-target effects," said Chu.
The ethics of gene editing
Gene editing hasn't been developed in a scientific ivory tower. Scientists and non-scientists alike have been challenging the technology before it was even feasible.
Scientists are in part self-monitoring themselves on the ethics front, and most agree that editing embryos is off-limits. The legal disaster around Jiankui's HIV-resistant babies set the precedent for how seriously countries take gene editing.
"It's pretty clear right now — you cannot edit germline DNA," said Chu.
Gene editing is here to stay, but not without pushback. For many, there's the issues of scientists "playing God". For others, there's a sense of discomfort, even disgust, with the idea of editing genes. After all, it interferes with nature.
But then again, wouldn't people want their disease or their child's disease to be fixed with gene editing? We have the tool to treat and even eradicate some diseases. What is more ethical — to use that technology to cure people, or to not use it at all?
Edited by: Clare Roth
(The above story first appeared on LatestLY on Apr 20, 2023 05:40 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website latestly.com).













 Quickly
Quickly