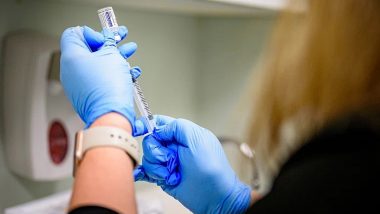
Mumbai, July 3: In recent, a picture has been going viral on the internet claiming that Bharat Biotech Vice-President Dr V.K. Srinivas became the first person in India to take COVID-19 vaccine developed by his team to fight the pandemic. However, Bharat Biotech refuted the claims and called it a routine procedural "blood draw for testing all production staff." Bharat Biotech Announces India's First COVID-19 Vaccine Candidate 'COVAXIN' with DCGI Approval for Human Clinical Trials.
"Dr V. K.Srinivas, Vice President, Bharat biotech, taking a clinical trial of Corona vaccine. He said that he is the first person in India to take vaccine developed by him & his team. Look at the confidence that they have in their product," the message claimed. COVID-19 Vaccine Update: ICMR Plans to Launch Coronavirus Vaccine Developed in Partnership With Bharat Biotech by August 15.
Viral Messages:
Dr V. K.Srinivas , Vice President, Bharat biotech, taking clinical trial of Corona vaccine.
He said that he is the first person in India to take vaccine developed by him & his team.
Look at the confidence that they have in their product.@JogulambaV pic.twitter.com/nQLK5SwyIg
— Sekhar Vasireddy (@SSVasireddy) July 3, 2020
@BharatBiotech Dr V. K.Srinivas , Vice President,Bharat biotech, taking Corona vaccine.clinical trial.
Immediately after taking the first dose he said that he is the first person in India to take vaccine developed by him and his team in Bharat Biotech.
(Is this true sir) via WA pic.twitter.com/h1C6huAO1Z
— kranthi kumar (@UrsKranti) July 3, 2020
Refuting the claims, Bharat Biotech said, "Certain images and messages being circulated on WhatsApp and other social media platforms have NOT been disseminated by Bharat Biotech. The image being circulated is a routine procedural blood draw for testing all production draw."
Bharat Biotech Tweet:
— BharatBiotech (@BharatBiotech) July 3, 2020
"We are working towards a safe and efficacious solution for COVID-19 and public health. Bharat Biotech will continue to abide to all necessary quality standards and safety guidelines towards vaccine clinical development," the message added.
Meanwhile, Bharat Biotech is the first Indian firm to receive approval to begin human clinical trials of its COVID-19 vaccine named Covaxin. The Indian Council of Medical Research (ICMR) has collaborated with the pharmaceutical company for fast-tracking clinical trials. ICMR Director General Balram Bhargava said that they targeting to release vaccine by August 15.
(The above story first appeared on LatestLY on Jul 03, 2020 09:16 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website latestly.com).













 Quickly
Quickly