London, October 15: The University of Oxford on Thursday announced scientists from the varsity's physics department have developed "an extremely rapid diagnostic test" that can detect coronavirus (COVID-19) in less than five minutes. According to the University of Oxford, the new test method can differentiate between high accuracy SARS-CoV-2, the virus that causes COVID-19 infection, and negative clinical samples, including other common respiratory pathogens such as influenza. COVID-19 Vaccine Development: Major Updates on Oxford-AstraZeneca, Johnson & Johnson, Sputnik V, Bharat Biotech & Sinopharm Candidates.
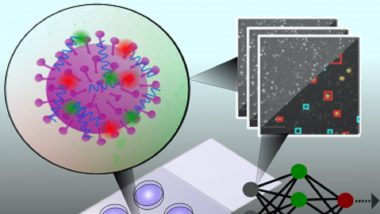
The method, which involved throat swabs, starts with the rapid labelling of virus particles in the sample with short fluorescent DNA strands. A microscope is then used to collect images of the sample, with each image containing hundreds of fluorescently-labelled viruses. Machine-learning software quickly and automatically identifies the virus present in the sample. The rapid testing method can be used for mass testing at airports, businesses and music concerts. India’s First CRISPR COVID-19 Test, Developed by Tata Group and CSIR-IGIB, Approved for Use by Govt.
"This approach exploits the fact that distinct virus types have differences in their fluorescence labelling due to differences in their surface chemistry, size, and shape," said the university. "Unlike other technologies that detect a delayed antibody response or that require expensive, tedious and time-consuming sample preparation, our method quickly detects intact virus particles; meaning the assay is simple, extremely rapid, and cost-effective," Professor Achilles Kapanidis, at Oxford’s Department of Physics, said.
Oxford University Scientists Develop Rapid COVID-19 Test:
Scientists from @OxfordPhysics have developed an extremely rapid diagnostic test for Covid-19 that detects and identifies viruses in less than five minutes.
Read more here >https://t.co/aY7ubgPz3G
— University of Oxford (@UniofOxford) October 15, 2020
The scientists are seeking investment to accelerate the translation of the rapid test method into a fully integrated device to be deployed as a real-time diagnostic platform. They hope to start product development in early 2021, and have an approved device available within 6 months of that time.
(The above story first appeared on LatestLY on Oct 15, 2020 04:35 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website latestly.com).













 Quickly
Quickly