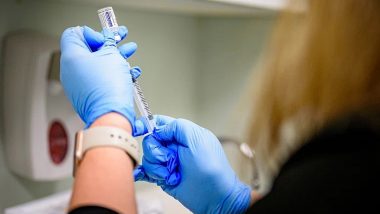
New Delhi, December 23: Hyderabad-based Bharat Biotech, which is developing the country's indigenous coronavirus vaccine named Covaxin in collaboration with the Indian Council of Medical Research (ICMR), has said its vaccine can generate antibodies that may persist for 6-12 months. Bharat Biotech also said the volunteers inoculated Covaxin during phase 1 and 2 trials did not show major side effects. The COVID-19 vaccine is currently undergoing phase 3 human clinical trials. COVID-19 Vaccine Latest Update: Bharat Biotech Again Applies for Emergency Use Authorization for Covaxin.
"After two doses, local and systemic adverse reactions observed in both vaccine groups were minimal, and the majority of them resolved within 24 hours of onset. No serious adverse events were reported in this study," the company stated in the paper uploaded on "medRxiv", a server which carries preprints of research work before it is peer-reviewed. Covaxin is an inactivated vaccine candidate. Amid New Coronavirus Strain Fears, 20 UK Returnees Test COVID-19 Positive in India So Far.
"The results from the phase 2 study show that both humoral and cell-mediated responses were observed. No neutralising antibody differences were observed between sexes and across age groups. BBV152 was well tolerated in both dose groups with no serious adverse events," Bharat Biotech said. The company on Wednesday again applied to the Drug Controller General of India for the emergency use authorisation.
Bharat Biotech had first applied for the emergency use authorisation of its vaccine on December 7 and presented its proposal, along with the interim safety and immunogenicity data of phase 1 and 2 clinical trials. The Central Drugs Standard Control Organisation's (CDSCO) expert panel had, however, recommended that the firm should present the safety and efficacy data from the ongoing Phase 3 clinical trial in the country for further consideration. (With IANS inputs)
(The above story first appeared on LatestLY on Dec 23, 2020 09:38 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website latestly.com).












 Quickly
Quickly