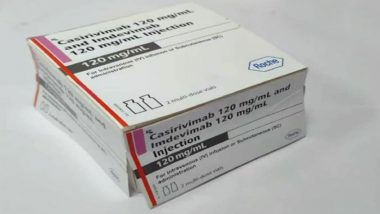
New Delhi, May 25: Drug major Roche India on Monday announced the launch of its first batch of antibody cocktail against COVID-19 in India. The antibody cocktail -- Casirivimab and Imdevimab -- is priced at ₹ 59,750 per dose. Roche's antibody cocktail was given to former US President Donald Trump when he contracted COVID-19 last year.
In a statement, the company said: "Each of the 1,200 mg dose of the drug contains 600 mg of Casirivimab and 600 mg of Imdevimab. The price of each dose will be ₹ 59,750. The maximum retail price for the multi-dose pack will be ₹ 1,19,500. Notably, each pack can treat two patients." Mucormycosis in India: Centre Allocates Additional 19,420 Vials of Amphotericin-B to States, UTs.
The drug will be marketed in India by Cipla and the second batch will be made available by mid-June.
"The first batch of the antibody cocktail (Casirivimab and Imdevimab) is now available in India while a second batch will be made available by mid-June. In total they can potentially benefit two lakh patients as each of the one lakh packs that will be available in India offers treatment for two patients," according to a joint statement issued by Cipla and Roche.
The drug will be available through leading hospitals and COVID treatment centres.
The Central Drugs Standards Control Organisation (CDSCO) had recently provided an Emergency Use Authorisation (EUA) for the antibody cocktail in India. Earlier, it has also received a EUA in the US and several EU countries.
"Roche is deeply committed to supporting the ongoing efforts to combat the COVID-19 pandemic, mitigate the deadly second wave and save lives. We are optimistic that the availability of Antibody Cocktail (Casirivimab and Imdevimab) in India can help in minimizing hospitalization, ease the burden on healthcare systems and play a key role in the treatment of high-risk patients before their condition worsens," said V Simpson Emmanuel, Managing Director and CEO, Roche Pharma India.
The antibody cocktail is to be administered for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age or older, weighing at least 40 kg) who are confirmed to be infected with SARS-COV2 and who are at high risk of developing severe COVID-19 disease and do not require oxygen.
The cocktail drug has been shown to help these high-risk patients before their condition worsens, reducing the risk of hospitalisation and fatality by 70 per cent.












 Quickly
Quickly