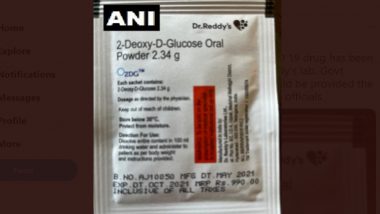
Hyderabad, Jun 28: Dr. Reddy's Laboratories Ltd on Monday announced the commercial launch of 2-deoxy-D-glucose (2-DG).
According to a press release issued by the city-based drug maker, Dr. Reddy's will supply the drug to major government as well as private hospitals across India.
In the initial weeks, the company will make the drug available in hospitals across metros and Tier 1 cities, and subsequently expand coverage to the rest of the country. 2-DG: DCGI Approves Anti-COVID-19 Oral Drug Developed by DRDO for Emergency Use.
2-DG manufactured by Dr. Reddy's has a purity of 99.5 per cent and is being sold commercially under the brand name 2DG.
The maximum retail price (MRP) of each sachet has been fixed at Rs 990, with a subsidized rate offered to Government institutions, it said.
2-DG, a oral drug was developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a laboratory of the Defence Research and Development Organisation (DRDO), in collaboration with Dr. Reddy's.
Dr. G Satheesh Reddy, Secretary, Department of Defence (R&D) and Chairman, DRDO said "We are pleased to have worked closely with our long-term industry partner Dr. Reddy's Laboratories, Hyderabad, for testing 2-DG as therapeutic application in treatment of COVID-19 patients. DRDO has been contributing in the fight against COVID-19 pandemic with its spin off technologies."
It can be administered only upon prescription and under the supervision of a qualified physician to hospitalised moderate to severe COVID-19 patients as an adjunct therapy to the existing standard of care.
Emergency use approval for anti-COVID-19 therapeutic application of the drug was granted on May 1, 2021.
Satish Reddy, Chairman, Dr. Reddy's, said "2-DG is yet another addition to our COVID-19 portfolio that already covers the full spectrum of mild to moderate and severe conditions and includes a vaccine. We are extremely pleased to have partnered with DRDO in our collective fight against the COVID-19 pandemic."












 Quickly
Quickly